By Amy Chang (Scripps College) and Chris Fang (Claremont McKenna College) [Edited by Lars Schmitz, as part of BIOL 167 “Sensory Evolution”, an upper division class at the W.M. Keck Science Department. Written for educational purposes only].
Contrary to what pop culture might lead you to believe, spectral sensitivity isn’t something that ghost hunters use to detect otherworldly specters—especially not the type that tend to lurk in abandoned houses in the woods, Scooby Doo-style. On this side of the scientific spectrum, spectral sensitivity is the detection of light based on the frequency or wavelength of its signal. Plainly put, it’s the ability of vision, an essential function for humans. But how about for spiders? Here, Zopf et al., 2013 pose the questions: How well can spiders see? And how much does it factor into their survival? Evolution and natural selection have always favored the development of senses that enhance a species’ survival, and in the process, removed traits that became extraneous. Historically, spiders were considered to rely mainly on their mechanosensory system for survival. Despite having eight eyes apiece, their visual senses have always been relegated to the background.

Figure 1. Dorsal view of the Cupiennius salei. (From: “The embryonic development of the central American wandering spider Cupiennius salei” (Figure 19f), by Wolff & Hilbrant, 2011, Frontiers in Zoology. Retrieved from https://commons.wikimedia.org/wiki/File:Cupiennius_salei_second_instar_-_1742-9994-8-15-19f.png)
This is the case with the American wandering spider Cupiennius salei (Figure 1). It was originally thought that this species of ctenid spider did not rely on its visual senses to function, instead mainly operating with its mechanosensory system to hunt and mate. However, recent studies have shown that the visual system of C. salei is in fact well-developed and may play a critical role in survival. A study by Fenk et al. (2010) showed that C. salei can hunt based on the visual stimuli that it detects. Another behavioral study conducted by Schmid (1998) concluded that C. salei use their eyes to selectively choose plants in which they can hide during the daytime, emerging at night to hunt. Their hunting grounds are the forests of Central America, an environment filled with many visual landmarks that they can potentially use for orientational behavior. Walla et al. (1996) provided a molecular basis for C. salei’s reliance on visual senses by identifying three photoreceptors in its eyes: one at 340nm (a UV receptor), one at 480nm (a blue light receptor), and one at 520nm (a green light receptor). Past research on C. salei has begun to suggest the importance of C. salei’s visual organs in daily function, but you might ask: how does it all fit together?
Spectral sensitivity requires that in order for an organism’s eye to discern light of different wavelengths, there must be specific opsin proteins that will detect and respond to specific wavelengths of light. Zopf et al. conducted behavioral tests to determine C. salei’s ability to navigate with its eyes in varied light conditions. Adult male spiders were placed in a darkened four-sided arena and oriented towards a black target on a wall (meant to represent potential prey) illuminated by monochromatic light. The experiments tested C. salei’s ability to accurately move towards the black target under two different light settings. Zopf et al. used monochromatic filters to adjust the amount and type of light transmitting into the arena; the first experimental setting had light transmission ranging from 40% to 80% and the second setting had an extremely low light transmission of 5%. To supplement the results from the behavioral tests with molecular evidence, Zopf et al. isolated RNA from the retinas of all eight eyes and used reverse transcription PCR (RT-PCR) to test for opsin genes in C. salei.
The results that Zopf et al. obtained indicated that C. salei does indeed possess spectral sensitivity within a certain range of wavelengths. In the first experiment, the spiders reacted to the black target in the setting of wavelengths between 389.9 nm and 654 nm, with around 82% of spiders yielding positive runs—i.e., running in a relatively straight line towards the target. In the second experiment, in which the transmission intensity was lowered, there was a 72% yield of positive runs between wavelengths of 448.5 nm and 598.6 nm. The pathways of C. salei movement in the experiments are pictured in Figure 2.
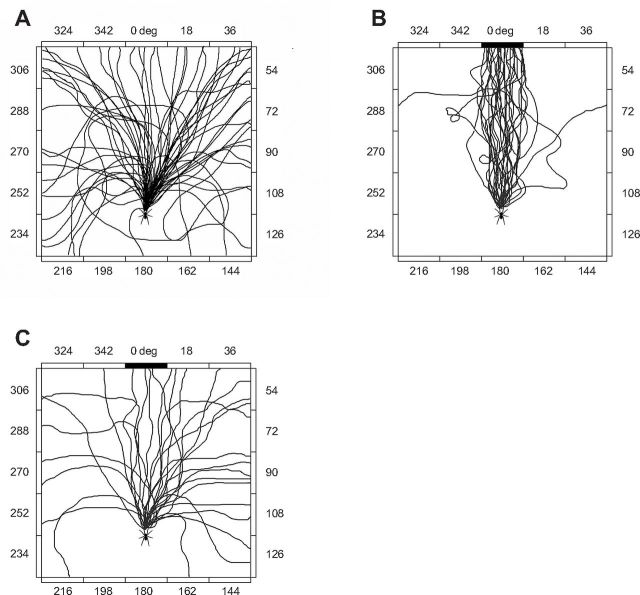
Figure 2. Movement pathways of C. salei in three different experiment settings: control (no target), 513.9 nm (within projected spectral sensitivity range), 695 nm (outside spectral range).
(From: “Spectral sensitivity of the ctenid spider Cupiennius salei” (Figure 1), by Zopf et al., 2013, The Journal of Experimental Biology. Retrieved from http://jeb.biologists.org/content/216/21/4103/F1.expansion.html)
In screening the C. salei transcriptomes, Zopf et al. found six opsin genes, three of which were identified as opsins of interest. A phylogenetic tree was then generated to depict the placement of C. salei’s opsins within a larger group of arachnids. The three critical opsins were grouped within arthropod visual opsins: Cs-Rh1 and Cs-Rh2 correlated with jumping spider opsins (Rh1 and Rh2), critical to detecting long to middle wavelengths, and Cs-Rh3 correlated with jumping spider opsin Rh3 for short and UV wavelengths (Figure 3).

Figure 3. Phylogenetic reconstruction of arthropod and onychophoran visual opsins. Arrowheads mark opsins of C. salei. Vertical bars mark spider opsins.
(From: “Spectral sensitivity of the ctenid spider Cupiennius salei” (Figure 2), by Zopf et al., 2013, The Journal of Experimental Biology. Retrieved from http://jeb.biologists.org.ccl.idm.oclc.org/content/216/21/4103/F2.expansion.html
So what do these results mean? The data that Zopf et al. collected implied that C. salei spiders possess an overall sensitivity to light wavelengths between 389.9 nm and 654 nm. Outside this range, spiders used their first pair of legs as feelers, indicating that C. salei could not see in an environment with light outside of its spectral sensitivity range (Schmid, 1997). An earlier study using electroretinogram (ERG) recordings revealed that C. salei may have a spectral sensitivity that ranges from 300 nm to 700 nm (Barth et al., 1993). The results of this paper affirmed that C. salei is indeed guided by visual input, although the range of 389.9 nm to 654 nm determined here is relatively narrow compared to the results of Barth et al.’s analysis, indicating some discrepancy in the extreme regions of C. salei’s visual range. Two receptors at 480 nm and 520 nm were confirmed to be integral to C. salei’s spectral sensitivity, which agreed with Barth et al.’s conclusions. Interestingly, the 340 nm UV receptor previously identified by Walla et al. was not confirmed here. The results of the second experiment indicated that in low light environments (~5% light transmission), C. salei possessed a spectral sensitivity range of 448.5 nm to 598.6 nm (green light). The experiment with RT-PCR confirmed that the three opsins found by Walla et al. are present in all eight eyes of C. salei; this provided the molecular evidence needed to corroborate the results of the behavioral tests. The Rh2 gene, which is linked to receptors that detected middle to longer wavelengths (450 nm to 570 nm), was shown to be the most abundant gene, emphasizing the importance of that range of spectral sensitivity to C. salei and implying a lesser importance for the disputed UV receptors.
So it turned out that the C. salei not only uses its adept mechanosensory system, but also its visual system. As shown by Zopf et al., within a wavelength range of 389.9 nm to 654 nm, C. salei can process visual input that enable it to detect prey at night and select dwelling plants to hide in during the day. This is especially useful since the forest environment that C. salei live in contain many landmarks, which provide many opportunities for visual signals to factor into fitness. And as Zopf et al. discovered, C. salei eyes are best equipped to detect green light, which correlates well with the color composition of its forest habitat. With the passage of time, organisms often evolve more complex systems for survival. Here, it’s evolutionary significant that C. salei depend on more than one sensory system, suggesting that C. salei evolved additional sensory complexity as time passed. So the next question becomes: Which sensory system appeared first in this lineage, mechanosensation or vision? It’s energetically costly to possess multiple well-developed sensory systems, and we’d like to know what makes it worthwhile for C. salei.
Overall, C. salei utilizes a formidable inventory of senses. Zopf et al.’s work confirmed previous findings and established grounds for further research that would provide us with insight into the continuing evolutionary traits of arachnids. We pose that a topic for future contention is the yet-unknown function of the UV receptors in C. salei. A possibility that was not considered in the paper is that these receptors may be a vestigial feature left over from ancestral species of ctenid spiders, a feature that aided the survival of older species but eventually became obsolete in present-day C. salei. To test this hypothesis, we could map opsin types on a phylogeny of ctenid (or all) spiders and determine if C. salei is unique in having UV receptors. If other species also possess the UV receptor gene, then it could be an inherited trait for some yet-undetermined function. We also suggest that another approach to the experiment could be to perform behavioral tests on the spider in a setting that more closely simulates C. salei’s natural environment—perhaps an arena made to look like a forest or one containing more sources for visual stimuli. This would diversify the C. salei’s experimental conditions, and its behavior could then be examined with greater surety of its accuracy.
But for now, if you happen upon a Cupiennius salei spider, just know this: it’s probably watching you with all eight eyes. Just don’t look up.
References
Barth, F. G. 2001 Sinne und Verhalten: aus Dem Leben Einer Spinne. Berlin; Heidelberg; New York, NY: Springer.
Barth, F. G., Nakagawa, T. and Eguchi, E. 1993 Vision in the ctenid spider Cupiennius salei: spectral range and absolute sensitivity. J Exp Biol 181, 63-79.
Fenk, L. M. and Schmid, A. 2010 The orientation-dependent visual spatial cut-off frequency in a spider. J Exp Biol 213, 3111-3117. (DOI: 10.1242/jeb.041939)
Schmid, A.1997 A visually induced switch in mode of locomotion of a spider. Z Nat Forsch 52, 124-128.
Schmid, A. 1998 Different functions of different eye types in the spider Cupiennius salei. J Exp Biol 201, 221-225.
Walla, P., Barth, F. G. and Eguchi, E. 1996 Spectral sensitivity of single photoreceptor cells in the eyes of the ctenid spider Cupiennius salei Keys. Zool Sci (Tokyo) 13, 199-202.
Zopf, L. M., Schmid, A., Fredman, D., Eriksson, B. J. 2013 Spectral sensitivity of the ctenid spider Cupiennius salei. J Exp Biol 216, 4103-4108. (DOI: 10.1242/jeb.086256)
You must be logged in to post a comment.